Since the FDA’s approval of the first recombinant protein in 1986, the number of approved biopharmaceuticals has grown constantly over the years, reflecting their increasing relevance in modern medicine. By 2021, the FDA approved the 100th monoclonal antibody product together with nine other new biologic drugs, accounting for 16% of the total number of drugs approved that year. This 35-year pattern of steady growth in the biopharmaceutical market reflects how these drugs are one of the most effective clinical treatment modalities for a broad range of diseases; as a result, they are increasingly being used in almost all branches of medicine.
Biotherapeutics are cutting-edge medicines which target specific molecules in the human body involved in the development of wide-spread diseases including cancer, immune and metabolic disorders. Mammalian cell lines, such as Chinese Hamster Ovary (CHO) cells and Human Embryonic Kidney 293 (HEK 293) cells, are the most common platforms used to manufacture recombinant DNA (rDNA) derived human therapeutic proteins. These cell lines, engineered to carry the gene that encodes the desired protein, are the most important assets that a biotech company can have. Therefore, companies are required to ensure the safety, purity and potency of these cell lines or cell substrates and to limit variability in the production process, in order to ensure a consistent final product.
Clonality: a key requirement for regulatory submission
A well-characterized clonally-derived cell line is not only a fundamental component of the biopharmaceutical manufacturing process but also a requirement for regulatory submission, most typically at the Investigational New Drug (IND) application stage. In fact, clonal derivation can impact a product’s critical quality attributes. Therefore, assurance of clonality in the production cell line is critical, and it should be confirmed early during cell line development. In fact, as a part of the overall control strategy, regulatory agencies require that all biomanufacturing cell lines be “cloned from a single cell progenitor” to ensure safety and consistent product quality. The United States (FDA) and the European Union (EMA) have different, though overlapping, guidelines for the manufacturing of biotherapeutics with regard to the clonal derivation of production cell lines.
Classic approaches for subcloning: Limited Dilution and Flow Cytometry (FACS)
In order to generate a clonally-derived cell line, a single precursor cell needs to be isolated, cultured and expanded. The traditional method to perform this subcloning step is limiting dilution (LD), which was developed at the beginning of the 1980s for cloning hybridoma cell lines in the production of monoclonal antibodies. Though it is a relatively inexpensive process, LD is very labor intensive, time consuming, slow and low in throughput; it is also highly variable between scientists and does not allow for documentation from a manual microscope. Moreover, the underlying statistical considerations are based on studies carried out with beads instead of cells and do not take into account the fact that cells do not behave like beads, as they tend to stick together. Therefore, even when performing a double round of cloning, complete assurance of genetic homogeneity may not be achieved.
Flow cytometry (FACS), which was originally developed as a research tool, now allows the detection and selection of cells based on the expression of defined cell surface markers. Indeed, due to this shear stress, many of the selected clones do not survive the selection procedure, and outgrowth rates are therefore low. Moreover, in order to be detected, cells need to be tagged with a fluorescent dye which could negatively influence the growth and expansion of the selected cell clone and also have undesired side effects in vivo.
Alternative cloning technologies: optimizing the isolation of single-cells
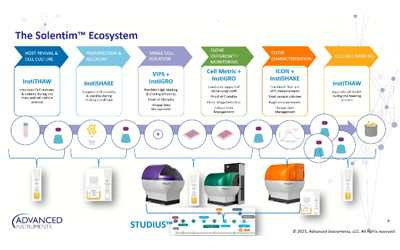
The need for gentler fluidics and the challenges associated with the LD method have driven the development of new low-pressure automated seeding methods. Depositing devices, such as Solentim VIPS® PRO, use image-based cell detection to deposit a single cell per well and immediately confirm these single cells by imaging them in situ in the well. Such devices achieve a seeding efficiency of 70-85%, varying with cell type, which is much higher than LD (30%).
|
Limiting Dilution |
FACS |
VIPS |
| Seeding efficiency |
30% |
99% |
70-85% |
| 96-well plates necessary to screen 1000 cells |
50 |
10 |
10 |
| Efficiency |
Low |
High |
High |
| Outgrowth probability |
High |
Low |
High |
Clonality assurance: whole well imaging
Due to the large volume of detailed information requested by regulators to evaluate the cloning process, biotech and pharma companies need to provide stringent proof of clonality. Therefore, they need valid data, such as images, to show what happened during cell bank generation. In addition, modern imaging technologies allow the monitoring of cell line outgrowth, which usually takes between 14 and 21 days for simple molecules like immunoglobulines and up to 28 days for more complex molecules. Solentim Cell Metric® provides whole well, high contrast imaging of the media-filled well to confirm the presence of a single cell at day 0 and daily imaging thereafter during the entire culture period.
Publications
Read more about assurance of clonality and how our instruments for Cell Line Development helped optimizing single cell cloning experiments around the world.
An innovative platform to improve asymmetric bispecific antibody assembly, purity, and expression level in stable pool and cell line development
Read the full paper
Recent advances in CHO cell line development for recombinant protein production
Read the full paper
Use of Solentim verified in-situ plate seeding (VIPS™) enhances single-cell cloning efficiency
Read the full paper
References
https://bioplanassociates.com/wp-content/uploads/2017/02/2019-FDA-Biopharmaceutical-Approvals-WP-22Jan2020.pdf
https://www.nature.com/articles/d41573-021-00079-7
https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2021-biological-license-application-approvals
https://pubmed.ncbi.nlm.nih.gov/28972297/