- Products
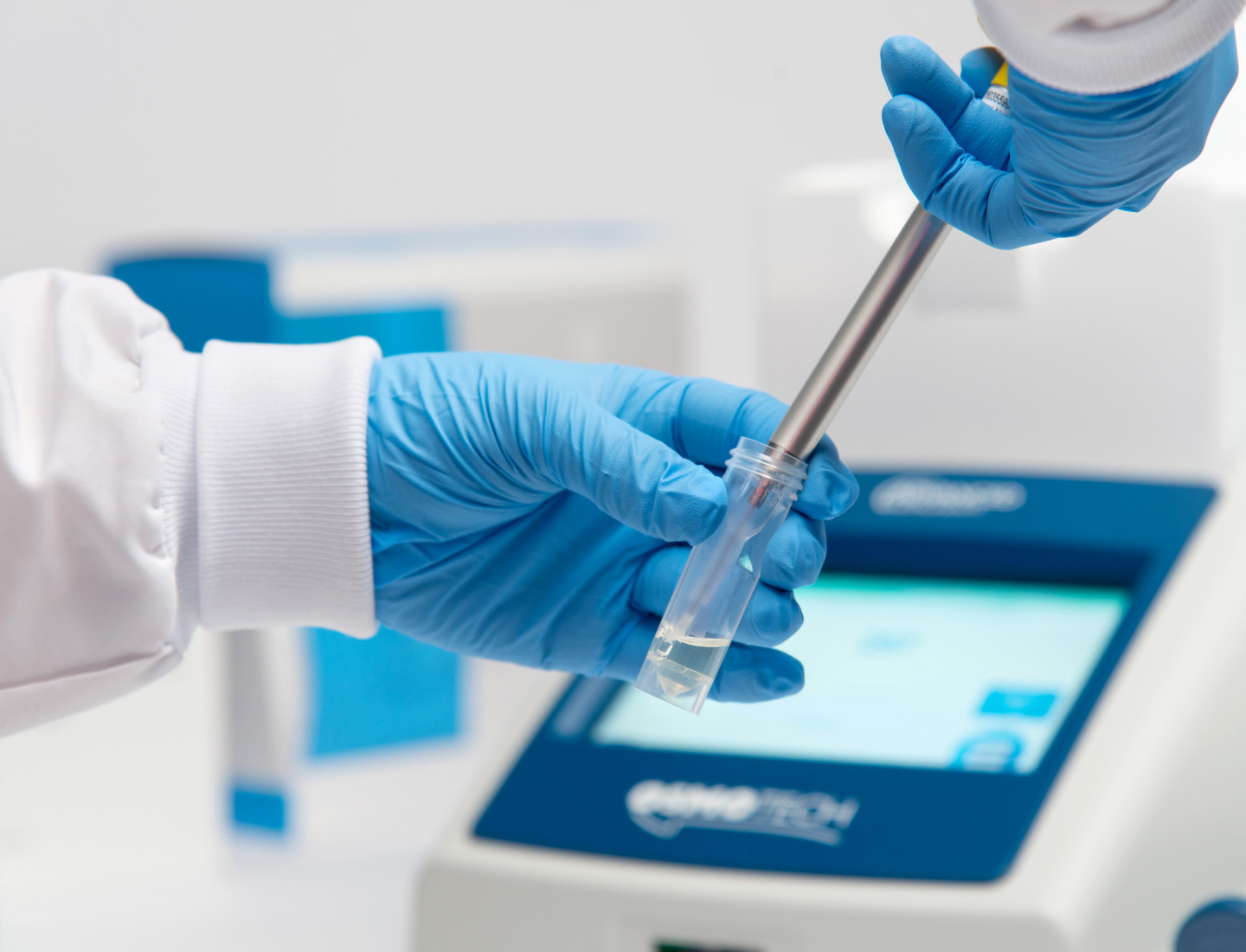
- Bioprocessing Osmometers
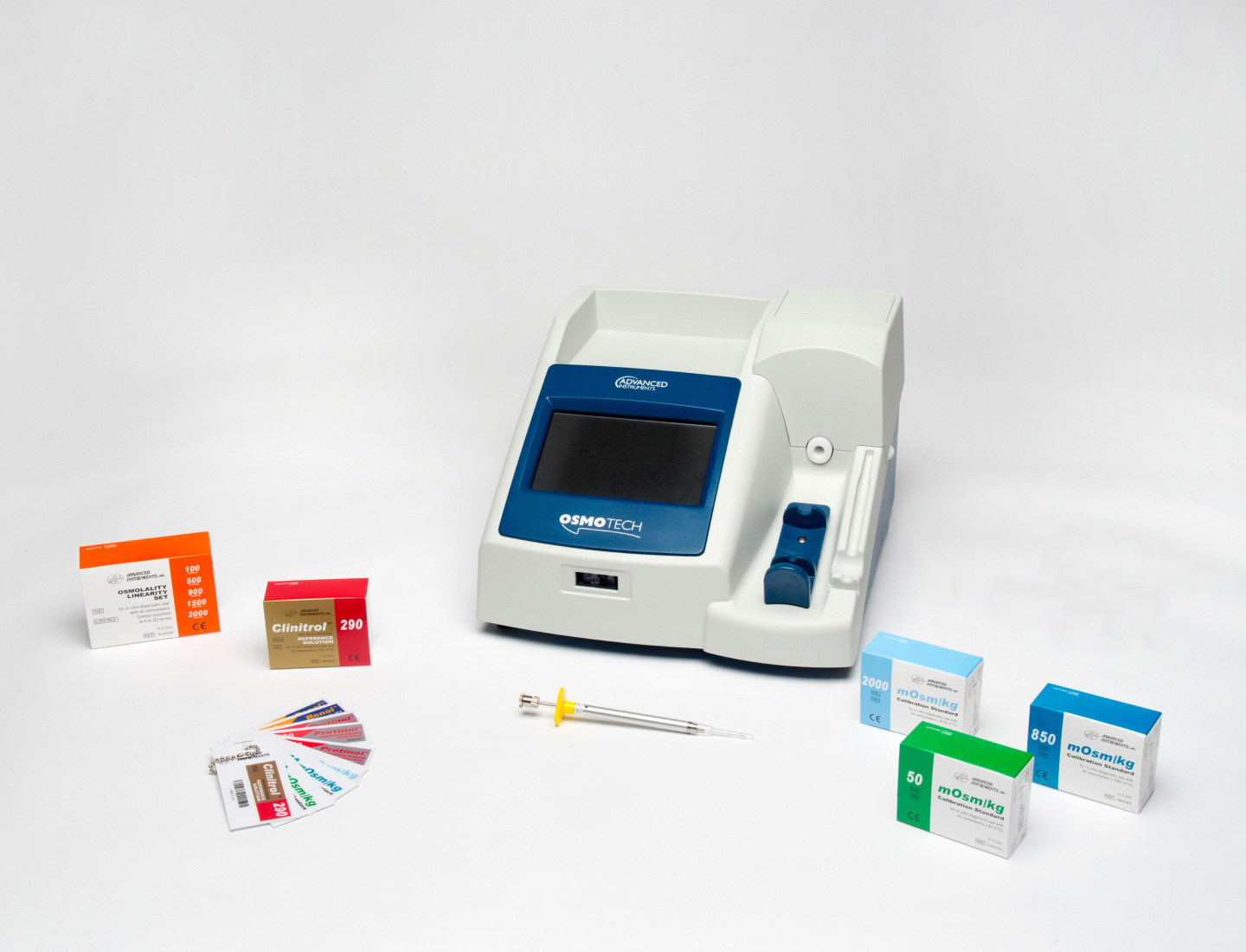
- Clinical Osmometers
- Cell Line Development
- Artel Liquid Handling Verification
- Anaerobic Jar Systems
- CSF Cell Counter
- Bilirubin Testing
- Dairy Testing
- Applications & Industries
- Bioprocessing
-
-
Compliance & Conformity
Our portfolio of instruments, consumables and services are designed to help you maintain regulatory guidelines, ensure data integrity, and meet GMP requirements.
-
- Clinical
-
-
Compliance & Conformity
Our portfolio of instruments, consumables and services are designed to support clinical lab regulations, standards and with HIPAA data security in mind.
-
- Food & Beverage
- Bioprocessing
- Service & Support
- Resources
- Company
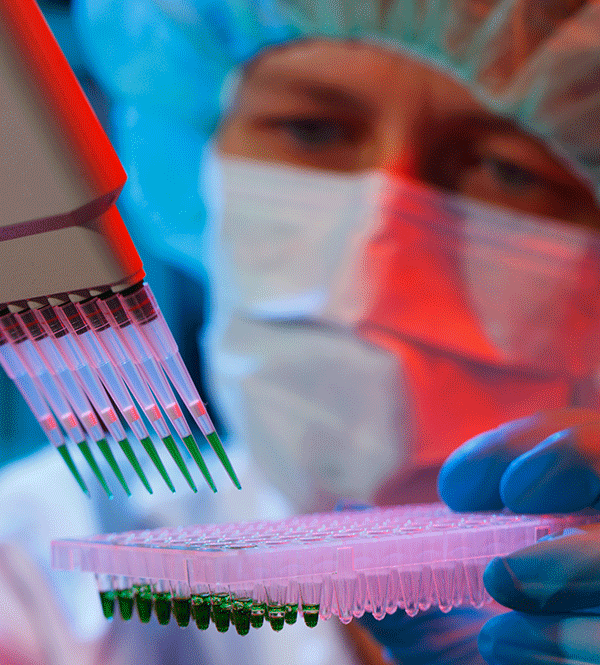
Monoclonal Antibody Vaccines
Osmolality testing should be performed on hybridoma cell media to ensure optimal antibody production rates. The rate of antibody production in hybridoma cells depends on the osmolality of the medium in which the cells are grown. The osmotic agent used to affect the osmolality levels also impacts Ab production.
- Osmolality testing should be performed on hybridoma cell media to ensure optimal antibody production rates.
- The rate of antibody production in hybridoma cells depends on the osmolality of the medium in which the cells are grown.
- The osmotic agent used to affect the osmolality levels also impacts Ab production.