Freezing point depression osmometers are the industry-preferred solution in clinical chemistry, pharmaceutical, and quality control labs. There are a number of logical reasons for this:
- All of the advantages and none of the disadvantages of other forms of osmometry – Membrane osmometry is limited by the lack of a perfect semi-permeable membrane; and vapor pressure osmometry is affected by volatile compounds exerting their own vapor pressure – a major drawback in the clinical chemistry lab setting.
- Freezing point measurements are fast and accurate – Test times are around 2 minutes and repeatability is in the range of ± 2 mOsm/kg H2O.
- Small sample size requirements – Typical sample volumes are in the µL range, this is advantageous for sample-limited and clinical lab settings.
- Simple to use – Requires very little knowledge or scientific background to operate systems and achieve good results.
- Limitless applicability and utility – Freezing point osmometers work well with almost any liquid matrix and are found in nearly every laboratory where osmolality testing is required.
- The most widely referenced and practiced technique for osmolality testing – Freezing point osmometers have been commercially available for over 50 years and are the most widely referenced technology for osmolality testing.
Types of Osmometers
An osmometer is a device for measuring the osmotic strength of a solution, colloid or compound. There are three major types of osmometers commercially available, each leveraging a particular colligative property to achieve their analytical results:
- Freezing Point Osmometers – determine the osmotic strength of solution by utilizing freezing point depression
- Vapor Pressure Osmometers – determine the concentration of osmotically active particles that reduce the vapor pressure of the solution
- Membrane Osmometers – measure the osmotic pressure of a solution separated by a semi-permeable membrane
| OSMOMETER TYPE |
ADVANTAGES |
DISADVANTAGES |
COMMENTS |
| Freezing Point Osmometry(FPO) |
- Performs rapid and inexpensive measurements
- Simple and reliable performance industry preferred FP method
- Small sample size (µl)
- Ideal for dilute biological and aqueous solution
|
- Samples must be of low viscosity
- May not be suitable for high molality colloidal solutions
|
FPO provides rapid and inexpensive results with the industry preferred freezing point method requires a small sample size and ideally suited for most biological and aqueous applications. |
| Vapor Pressure Osmometry (VPO) |
- Performs rapid and inexpensive measurements
- Small sample size (µl)
- The ideal of dilute biological and aqueous solutions
|
- Less accurate than FPO
- Cannot be used for volatile solutes like alcohols or other organic solvents
- Not ideally for high molality or colloidal solutions
|
VPO provides fast, inexpensive measurements requiring a small sample size although not as fast or reliable to VPO limiting its utility in many application |
| Membrance Osmometry (MO) |
- Provides potentially unlimited direct measurement of osmotic pressure and solution osmolatity
- Good for colioidat solution
- No limitation on sample concentration
- Can determine MW of macromolecules
|
- Time-consuming and difficult to operate
- Requires large sample volume
- Not applicable for small molecules and aggressive solvents due to membrance porosity and compatibility
- Irreproducible results due to clogging of membrance pores
|
MO provides a direct measurement of osmolatity and is suitable for high molality and colloidal samples, long analysis time, and requires a large sample volume. Not applicable for small molecule applications |
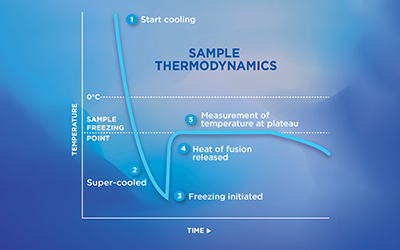
Theory of Freezing Point Depression
Advanced® Osmometers use the industry-preferred freezing point method to determine the osmolality of an aqueous-based solution. When a solute (particles) is dissolved in a solvent (water), the freezing point of that solution is lower than that of the solvent alone. As more solute is added, the freezing point decreases further. By precisely measuring the freezing point of the solution, the osmolality, or concentration, can be determined. Freezing point measurement is non-specific; that is, it will not tell you what the particle is, nor will it tell you the size or shape of the particle. The freezing point depends only on the number of particles in solution.